Rethinking by repositioning..
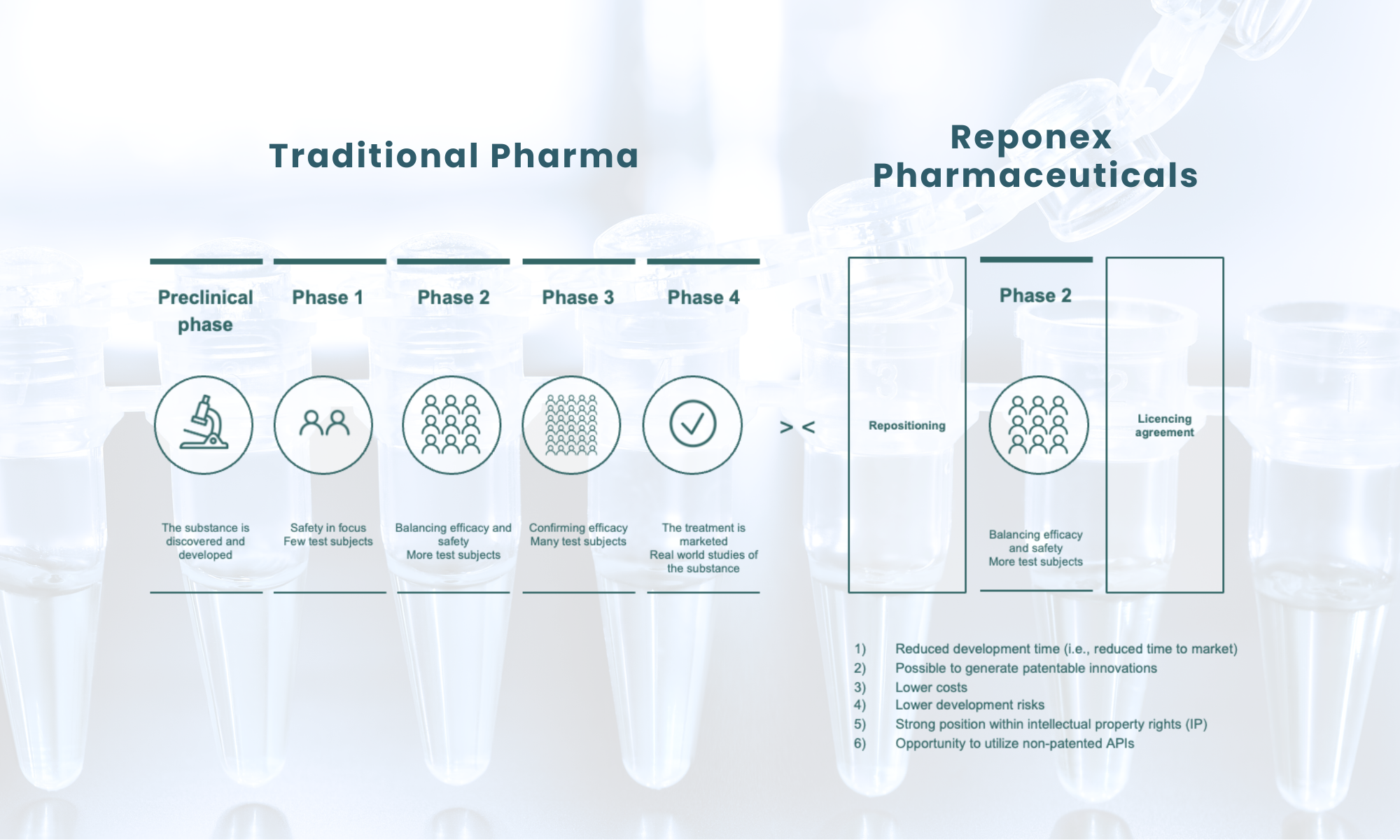
Reponex Pharmaceuticals identifies novel applications for active substances originally intended for other treatments. This strategy involves repurposing substances beyond their initial designated and registered use. The primary advantage is that the basic toxicity and adverse effect profiles of these substances are already well-documented and understood. The advantage of this is that the active substance’s basic toxicity and adverse effect profile are already known and described.
Repositioning has the potential to affect a significant number of patients, where there is currently an unmet medical need. When repositioned treatments result in an improved efficacy, safety, and or cost-saving effect both the patients and the drug developers reap the benefits. Pharmaceutical companies can save both money and time in development by streamlining validation studies without the need to repeat human safety studies, giving patients faster access to new treatment paradigms aimed at treating their diseases.
Through the innovative reuse of existing knowledge, Reponex seeks to execute its clinical development programs to the fullest possible extent and thereby achieve a low project risk. By combining effective drug development strategies, i.e. by repositioning in combination with a new route of administration, and in some cases by combination with various other active substances that act synergically on various aspects of the disease, the developments seek to achieve a potent therapeutic effect.
Our mission & Vision
Our business model
It is Reponex’s ambition to create value through the company’s sustaining platform by bringing the clinical programs to a clinical phase II stage with securing IP at which the effects of the drug candidates can be documented with relevant clinical data.
Reponex is an organizational efficient company with an aggressive commercial outsourcing strategy to be as agile as possible, to meet complex and continual changes in the pharma industry. The strategy creates a cost efficient and flexible way to create relevant humane ressources fast, which is considered a key factor and drives success.
Reponex drug development is based on a combination of:
- Repositioning existing APIs (active pharmaceutical ingredients).
- Clinical development strategy
- Regulatory development strategy
- Patent protection (IP)
- Outsourcing
Our Pharmaceutical approach
Strategy
It is Reponex’s clinical strategy to establish collaborations with internationally leading institutions and hospitals in combination with the best experts within the specific clinical areas, thereby executing Reponex’s clinical development in close interaction with the latest knowledge and research, which regularly results in publications that directly or indirectly validate the merit of Reponex’s clinical development programs. This means that Reponex conducts its clinical programs in a relevant scientific environment that has direct access to patients and continuously retrieves references on the latest knowledge and research in the field.

